 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 제품번호 | 종 | 제품 설명 | 구조 | 순도 | 특징 |
|---|---|---|---|---|---|
| FXI-C52H3 | Rhesus macaque | Rhesus macaque Coagulation factor XI Protein, His Tag (SPR verified) |  |
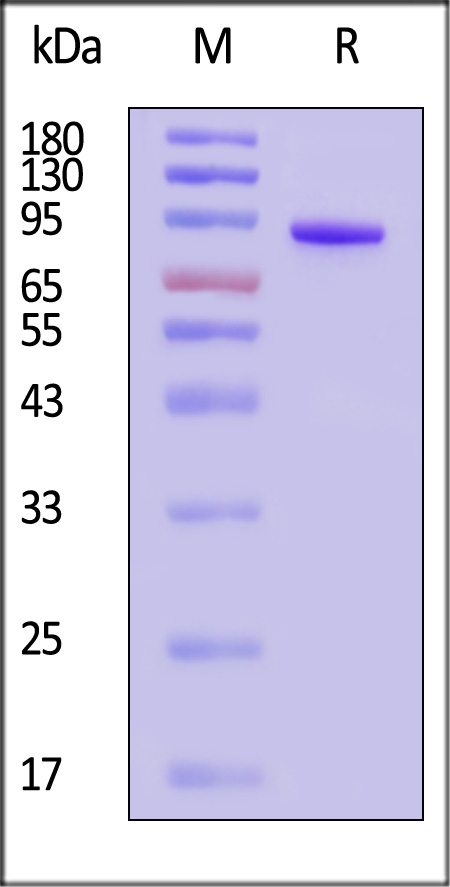
|
|
| FXI-H52H5 | Human | Human Coagulation factor XI / FXI Protein, His Tag (active enzyme) |  |
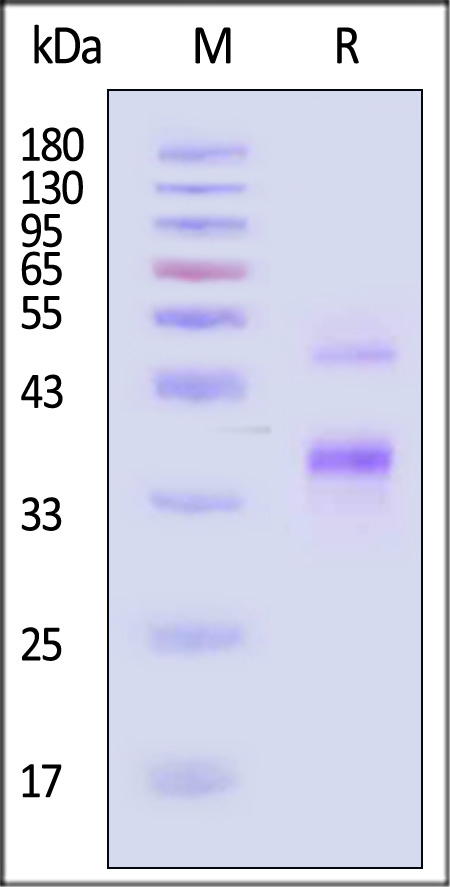
|
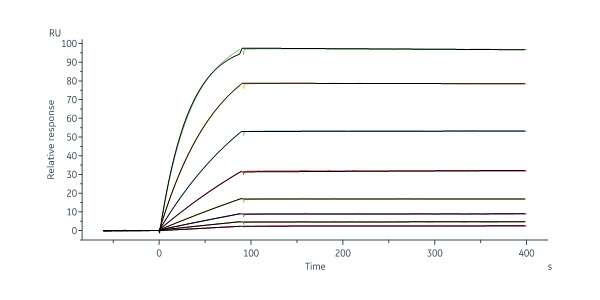
Abelacimab captured on Protein A Chip can bind Rhesus macaque Coagulation factor XI, His Tag (Cat. No. FXI-C52H3) with an affinity constant of 22.4 pM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Factor XI concentrate (factor XI deficiency), CSL Behring | Approved | Csl Behring Llc | Australia | Factor XI Deficiency | Csl Behring Llc | 2014-07-01 | Factor XI Deficiency | Details | ||
| Human coagulation Factor XI (LFB) | Approved | Lfb Biotechnologies Sas | Hemoleven | EU | Factor XI Deficiency | Lfb Biotechnologies Sas | 1999-07-01 | Factor XI Deficiency | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Factor XIa Inhibitor (Bayer) | Factor XIa Inhibitor (Bayer) | Phase 1 Clinical | Blood Coagulation Disorders | Details | |
| SKB-336 | SKB-336 | Phase 1 Clinical | Sichuan Kelun-Biotech Biopharmaceutical Co Ltd | Thromboembolism | Details |
| BMS-262084 | BMS-262084 | Phase 1 Clinical | Bristol-Myers Squibb Company | Thrombosis | Details |
| IONIS-FXIRX | FXI-ASO; ISIS-FXIRX; BAY-2306001; IONIS-FXIRX; ISIS-404071; ISIS-416858 | Phase 2 Clinical | Ionis Pharmaceuticals Inc | Venous Thromboembolism; Thrombosis | Details |
| ONO-7269 | ONO-7269 | Phase 1 Clinical | Ono Pharmaceutical Co Ltd | Cerebral Infarction | Details |
| SHR2285 | SHR-2285 | Phase 2 Clinical | Jiangsu Hengrui Medicine Co Ltd | Embolism; Venous Thromboembolism; Thrombosis | Details |
| ONO-7684 | ONO-7684 | Ono Pharmaceutical Co Ltd | Details | ||
| BMS-962212 | BMS-962212; BMS-96221202; BMS-962212-02 | Bristol-Myers Squibb Company | Details | ||
| Osocimab | BAY-1213790 | Phase 2 Clinical | Bayer AG | Thromboembolism; Uremia; Kidney Failure, Chronic | Details |
| BAY-1831865 | AB-012; BAY-1831865 | Phase 1 Clinical | Aronora Inc | Thrombosis | Details |
| Xisomab 3G3 (Oregon Health & Science University) | AB-022; AB-023 | Phase 2 Clinical | Oregon Health & Science University | Solid tumours; Multiple Myeloma; Lymphoma | Details |
| Ir-CPI | Ir-CPI | Phase 1 Clinical | Bioxodes SA | Details | |
| Milvexian | BMS-986177; JNJ-70033093; JNJ-3093 | Phase 2 Clinical | Bristol-Myers Squibb Company | Kidney Diseases; Stroke; Thromboembolism; Ischemic Attack, Transient; Thrombosis | Details |
| SAL-0104 | SAL-0104; SAL0104 | Phase 1 Clinical | Suzhou Genemen Biotech Co Ltd | Embolism; Myocardial Infarction; Venous Thromboembolism; Stroke; Ischemic Attack, Transient; Cardiovascular Diseases; Venous Thrombosis | Details |
| KN060 | KN-060 | Phase 1 Clinical | Thromboembolism | Details | |
| EP-7041 | EP-7041; HSK36273; HSK-36273; EP7041 | Phase 2 Clinical | Exithera | Blood Coagulation Disorders; Coronavirus Disease 2019 (COVID-19); Thrombocytopenia; Thrombosis | Details |
| REGN7508 | REGN7508 | Phase 1 Clinical | Regeneron Pharmaceuticals Inc | Details | |
| NIP-003 | NIP003 | Phase 1 Clinical | National Institutes Of Pharmaceutical Research And Development Co Ltd | Thromboembolism; Venous Thrombosis | Details |
| Abelacimab | MAA-868; NOV-12 | Phase 3 Clinical | Novartis Pharma Ag | Pulmonary Embolism; Venous Thromboembolism; Stroke; Atrial Fibrillation; Venous Thrombosis | Details |
| Fesomersen | ION-957943; IONIS-FXI-LRx; BAY-2976217 | Phase 2 Clinical | Ionis Pharmaceuticals Inc | Nephrosis; Thromboembolism | Details |
| REGN-9933 | REGN-9933 | Phase 2 Clinical | Regeneron Pharmaceuticals Inc | Venous Thromboembolism | Details |
| BMS-986209 | BMS-986209 | Phase 1 Clinical | Bristol-Myers Squibb Company | Details | |
| Asundexian | BAY-2433334 | Phase 2 Clinical | Bayer AG | Myocardial Infarction; Thromboembolism; Stroke; Atrial Fibrillation; Hepatic Insufficiency | Details |
| MK-2060 | MK-2060 | Phase 2 Clinical | Merck Sharp & Dohme Corp | Uremia; Kidney Failure, Chronic; Thrombosis | Details |
This web search service is supported by Google Inc.
